Products
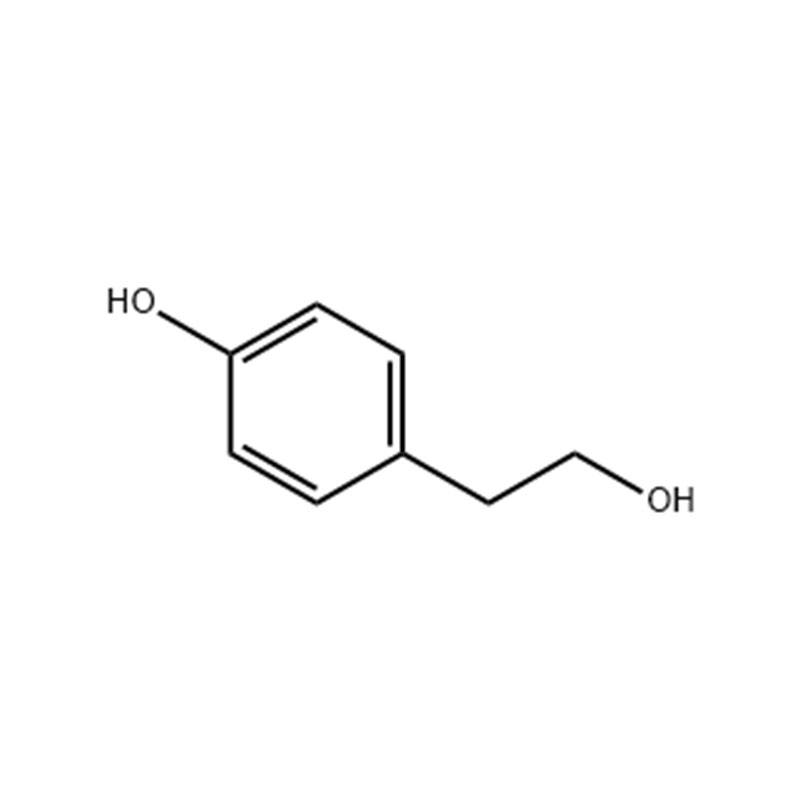
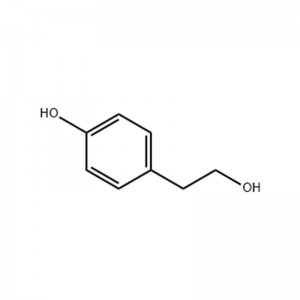
Tyrosol
Structural Formula
Physical
Appearance: white crystalline powder
Density: 1.0742
Melting point: 89-92°C
Boiling point: 195°C
Refractive index: 1.5590
Safety Data
Hazard category: General goods
Application
Tyrosol is an intermediate of petalol and is mainly used to synthesize the cardiovascular drug metoprolol. It is used to chelate zero valent iron for catalytic oxidation of organic acids in olive mill wastewater.
p-Hydroxyphenethylalcohol, p-Hydroxy-benzeneethanol, also known as 4-Hydroxyphenethyl alcohol, beta-(4-hydroxyphenyl)ethanol, 2-(4-hydroxyphenyl)ethanol, and Tyrosol. Hydroxyphenyl)ethanol, 2-(4-hydroxyphenyl)ethanol, commonly known as Tyrosol. It is white crystal at room temperature, soluble in alcohol and ether, slightly soluble in water. It is flammable and has the risk of burning when exposed to high temperature, open flame or oxidizing agent. Can irritate the eyes, skin and respiratory system, lack of data on toxicity, its toxicity can be referred to phenol. Mainly used in the synthesis of cardiovascular drug Medocin. Molecular formula is C8H10O2.
Toxicity and environmental impact: can irritate the eyes, skin and respiratory system, lack of toxicity data, its toxicity can be referred to phenol. Should be concerned about the adverse effects of production process waste and by-products on the environment.
Packing, transportation and storage: p-hydroxyphenylethanol is packed in cardboard drums lined with two layers of polyethylene film or kraft paper, 25KG/ drum. Keep away from oxidizing agent, fire and heat source, store in a sealed place and put it in a ventilated, cool and dry environment.
Manufacturing methods: (1) Hydroxyacetophenone as raw material, oxidized with aliphatic nitrile, then hydrolyzed to obtain p-hydroxyphenyl glyoxal, which can be obtained by reduction of p-hydroxyphenylethanol.
(2) obtained by oxidation with β-aminophenethyl alcohol as raw material.