Products
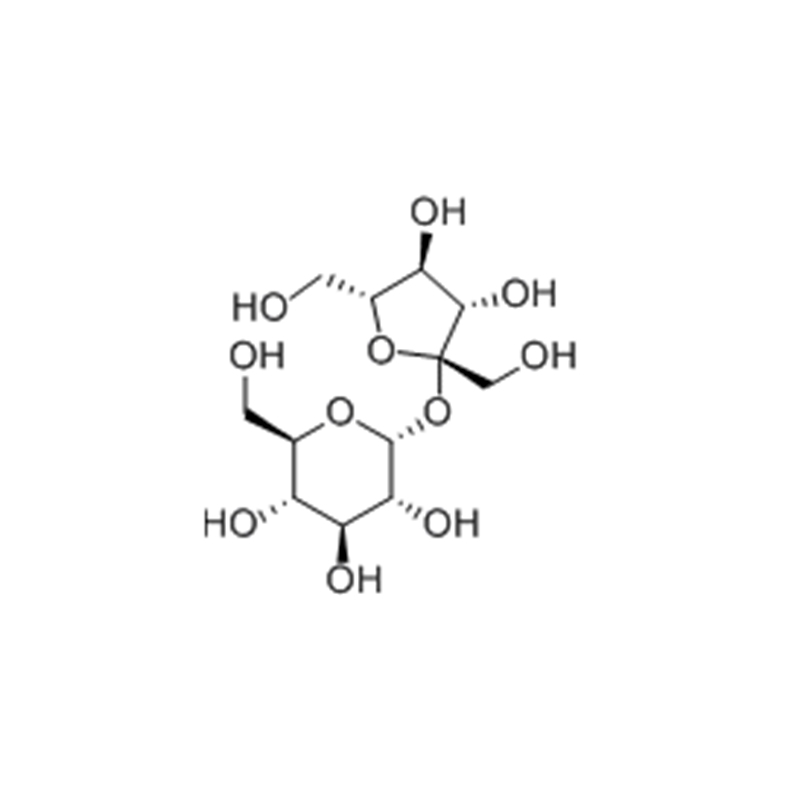
Sucrose
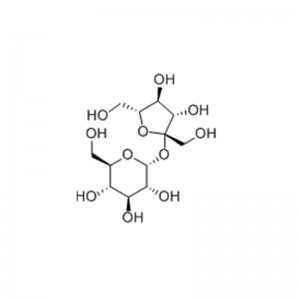
Structural Formula
Physical Properties
Appearance: White crystalline odorless solid
Density: 1.5805
Melting point: 185-187°C (lit.)
Boiling point: 397.76°C (rough estimate)
Specific Rotation:67 º(c=26, in water 25 ºC)
Refractivity:66.5 °(C=26, H2O)Flash point 93.3°C
Solubility:H2O: 500 mg/mL
Acidity coefficient(pKa):12.7(at 25°C)
PH:5.0-7.0 (25°C, 1M in H2O)
Safety Data
Belongs to the dangerous goods
Customs code:2938909090
Export Tax Refund Rate(%):9%
Application
Sucrose is widely used in food, cosmetics and medicine, and is also used as a standard for analysis and detection. It can be used to prepare citric acid, caramel, invert sugar, transparent soap, etc. It can inhibit the growth of bacteria at high concentration, and can be used as tablet excipient of preservative and antioxidant in medicine. The reagent sucrose is used for the determination of 1- naphthol, the separation of calcium and magnesium and the preparation of biological culture medium.
Sucrose, the main component of table sugar, is a type of disaccharide, which consists of a molecule of the hemiacetal hydroxyl group of glucose and a molecule of the hemiacetal hydroxyl group of fructose condensed with each other and dehydrated. Sucrose is sweet, odorless, soluble in water and glycerol, and slightly soluble in alcohol. It is spinogenic, but has no photochromic effect. Sucrose is almost universally found in the leaves, flowers, stems, seeds and fruits of the plant kingdom. It is particularly abundant in sugarcane, sugar beet and maple sap. Sucrose has a sweet taste and is an important food and sweet flavoring. It is divided into white sugar, brown sugar, rock sugar, rock sugar, and coarse sugar (yellow sugar)
Physical properties
Sucrose is very soluble in water, and its solubility increases with the increase of temperature, and it does not conduct electricity when dissolved in water. Sucrose is also soluble in aniline, azobenzene, ethyl acetate, amyl acetate, melted phenol, liquid ammonia, mixture of alcohol and water and mixture of acetone and water, but it is insoluble in organic solvents such as gasoline, petroleum, anhydrous alcohol, trichloromethane, carbon tetrachloride, carbon disulfide and turpentine. Sucrose is a crystalline substance. The specific gravity of pure sucrose crystals is 1.5879, and the specific gravity of sucrose solution varies depending on the concentration and temperature. The specific rotation of sucrose is +66.3° to +67.0°.
Chemical properties
Sucrose and sucrose solutions under the action of heat, acid, alkali, yeast, etc., produce a variety of different chemical reactions. The reaction results not only in direct loss of sucrose, but also in the production of substances that are harmful to sugar production.
When crystallized sucrose is heated to 160°C, it will thermally decompose and melt into a thick and transparent liquid, and then recrystallize when cooled. Heating time is extended, sucrose is decomposed into glucose and defructose. At a higher temperature of 190-220°C, sucrose will be dehydrated and condensed into caramel. Further heating of caramel produces carbon dioxide, carbon monoxide, acetic acid and acetone. Under humid conditions, sucrose decomposes at 100°C, releasing water and darkening the color. When a sucrose solution is heated to boiling at atmospheric pressure for a long time, the dissolved sucrose slowly decomposes into equal amounts of glucose and fructose, i.e., conversion occurs. If the sucrose solution is heated above 108℃, it will be hydrolyzed rapidly, and the greater the concentration of sugar solution, the more significant the hydrolysis effect. The metal material used in the boiling vessel also has an effect on the rate of sucrose conversion. For example, the conversion of sucrose solution in copper vessels is much larger than in silver vessels, and glass vessels have little effect.